Potassium hydroxide
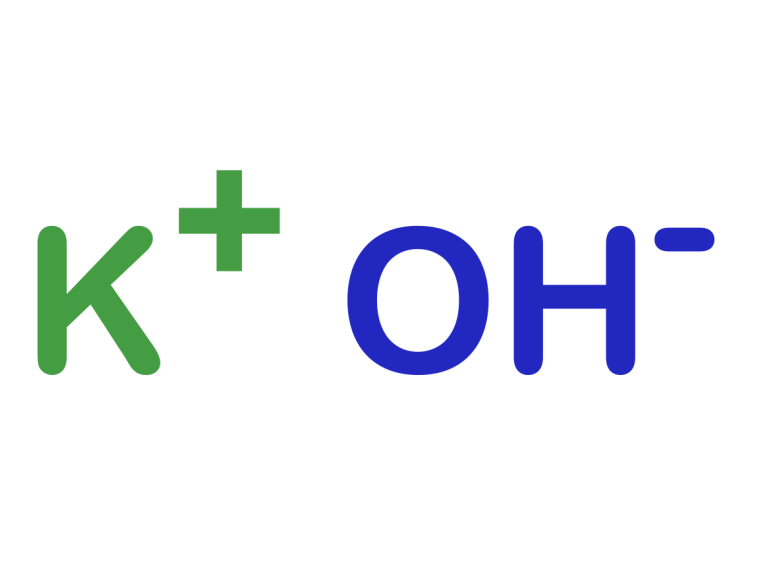
Potassium hydroxide (KOH), also known as caustic potash, is an extremely versatile and important chemical compound with a wide range of applications in various industries. It is an alkaline compound commonly used in the manufacture of soaps, detergents and other cleaning products, as well as fertilisers, batteries and pharmaceuticals. It seems like a very simple compound, but it's importance in many critical chemical processes made me want to learn more about it. During my research I came across some environmental facts that I found exciting and worthy of a blog post.
The importance of potash
As mentioned above, potassium hydroxide (KOH) is an essential chemical compound that has a wide range of uses in various industries. One of the most important uses is in the manufacture of soap and detergents. When mixed with fatty acids, potassium hydroxide reacts to form soap. Soap is, of course, a cleaning agent that effectively removes dirt, grease and other impurities from surfaces. The history of potassium soap can be traced back to ancient civilisations such as the Egyptians and Romans, who used a type of soft soap made from ashes and animal fats. However, the modern production of potassium soap began in the 19th century, when the use of potassium hydroxide became more widespread. Potassium soap was traditionally used for laundry and cleaning purposes, but today it is also used in the manufacture of other products such as cosmetics and industrial lubricants. Potassium soap (made from KOH) is known as 'soft soap', while sodium soap (made from NaOH) is known as 'hard soap'. Potassium soap dissolves more easily in water and produces a lot of foam, making it ideal for use in liquid soaps, shampoos and other cleaning solutions. Sodium soap, on the other hand, is harder and does not dissolve easily in water. It is typically used in bars of soap and other soap products. Another difference is that potassium soap has a higher pH than sodium soap, which makes it less harsh on the skin and may be more suitable for people with sensitive skin.
Potassium hydroxide is also used in the manufacture of fertilisers to improve soil quality and fertility. Potassium is involved in important plant functions such as stomatal movement, water balance, disease resistance, cold tolerance and the overall quality of fruit and vegetables. Potassium hydroxide (KOH) can also be used to raise the pH of acidic soils, making them more suitable for plant growth. The history of potassium hydroxide (KOH) as a fertiliser dates back to the 19th century, when scientists first began to understand the importance of potassium for plant growth. Early uses of potassium hydroxide as a fertiliser were limited because the production process was expensive and not widely available (more on this later). As technology advanced and new production methods developed, the use of KOH as a fertiliser became more efficient and cost-effective. Potassium hydroxide (KOH) is still used as a potassium fertiliser today, although it is not as widely used as other forms of potassium fertiliser such as potassium chloride (KCl) or potassium sulphate (K2SO4). Potassium hydroxide is mostly used to make other fertilisers and chemicals, such as potassium carbonate (K2CO3). One of the main reasons for this is the dangers associated with potassium hydroxide. Potassium hydroxide is a strong base and can cause chemical burns if it comes into contact with the skin or eyes. It is also highly reactive and can release heat when mixed with water or other acidic compounds. In fact, it is so reactive that it can be used to make a type of gunpowder known as "potassium nitrate gunpowder" or "black powder" (KNO3).
_Black powder KNO3. Image by Lord Mountbatten via Wikimedia._
KOH is also used in another everyday item: Alkaline batteries. Alkaline batteries are a type of primary cell battery that uses an alkaline electrolyte, typically potassium hydroxide (KOH), to provide a longer shelf life and higher energy density than conventional batteries. The KOH electrolyte is dissolved in water and placed in the cells of the battery along with the anode and cathode. The anode and cathode are usually made of zinc and manganese dioxide respectively. However, it is less commonly used in lithium batteries, where the electrolyte is a lithium salt dissolved in an organic solvent. However, it is still used in some manufacturing processes of lithium batteries. Potassium hydroxide is used as a solvent or reagent to make lithium cobalt oxide (LiCoO2), which is used as a cathode in some lithium-ion batteries.
There are countless other applications in the chemical and pharmaceutical sectors. Most importantly, KOH is used to balance the pH of a solution or is used as an extractant. It is also essential for the oil and gas industry, such as for extracting crude oil from bitumen sand. It is also involved in the production of important chemicals such as potassium carbonate, potassium permanganate or as a base and catalyst in organic synthesis reactions.
The history of potassium hydroxide
The history of potassium hydroxide production dates back to ancient times, with the first recorded production of potassium hydroxide taking place in Ancient Egypt around 2500 BC. At that time, potassium hydroxide was produced by burning wood in a process known as "causticization", which involved heating wood to high temperatures to produce a solution of potassium hydroxide. This process was labour intensive and required large quantities of wood, making it an inefficient method of production. This process, which is also called 'lye leaching', was the only significant source of KOH for a very long time. In general it works like this:
- Collection of wood ash: Wood ash is collected from wood fires.
- Leaching: The wood ash is mixed with water to form a slurry and then left to leach for a period of time. The leaching process extracts potassium hydroxide from the wood ash and forms the 'potash lye'.
- Evaporation: The potash liquor is then evaporated to concentrate the potassium hydroxide.
- Crystallisation: The concentrated solution is then cooled and allowed to crystallise, resulting in the formation of potassium hydroxide crystals.
- Purification: The crystals are then repeatedly washed with hot water and dried to remove impurities.
- Sieving: The washed and dried crystals are sieved to remove impurities and homogenise the product.
According to this video from Veritasium, potash was the most important chemical compound produced by early American settlers. In 1788, there were 250 potash works in the state of Massachusetts alone. Demand for potash was so high that forests across Europe and the USA were decimated simply to burn hardwoods and produce potash. How sustainable is that? In addition to large-scale deforestation, there are a number of other negative environmental impacts associated with the production of KOH by caustic leaching, including air pollution, water pollution, chemical waste and soil erosion as a result of deforestation. In the late 19th century, potassium was mined in Germany, which had a monopoly until the end of the First World War. Potassium is found in minerals such as halite, sylvite or carnallite. These minerals can be ground and used directly as fertiliser or further processed to extract KOH. Today, potash mines can be found in many places around the world, with the largest producers being Canada, Russia and Belarus. Sometimes potash mines are used in combination with a production method called "in situ leaching". One such example is the Kane Creek potash mine in Utah, USA. This used to be a conventional mine, opened in 1963. However, after a mining accident, it was converted to a system that combines solution mining and solar evaporation. River water is pumped into the mine which dissolves the potash. Then, the brine solution is pumped to above-ground evaporation ponds and the crystallized potassium hydroxide havested.
 Potash evaporation ponds at the Intrepid mine near Moab, Utah. Image by Doc Searls via Wikimedia.
Potash evaporation ponds at the Intrepid mine near Moab, Utah. Image by Doc Searls via Wikimedia.
When I first heard about the traditional method of producing potassium hydroxide, which was simply to burn hardwood and leach the ash, I was astonished and thought it was the most unsustainable production process in history. On second thought, it makes sense. Potassium hydroxide is an important chemical product for our civilisation and potassium minerals are mostly found at depths of 1000m, which was too deep for early mining technologies. It also made me wonder, what is the equivalent of today's time? I'm pretty sure it would be the use of fossil fuels, which have to be extracted from the earth and are associated with a myriad of negative environmental and social impacts. After all, the sun sends a lot of energy and 173,000 terawatts of solar energy are constantly hitting the earth. By comparison, the United States consumed 3,930 terawatt hours of electricity in 2021. So why even bother burning fossil fuels? I guess we all know the reasons, but in the year 2250, people might just be as surprised and might also write a blog article about it.